
The more efficient plant varieties, the more precisely they should be mobilized to grow faster, but also the quality of the crop should be influenced. This goal can be achieved by thoroughly understanding the nutritional requirements and fertilization needs of plants, while observing basic laws. Get to know the most important ones and learn more about mineral fertilizers.
The most important of them is the von Liebig's law, which informs that "the size of plant yield depends on the nutrient present in the soil in the smallest amount".
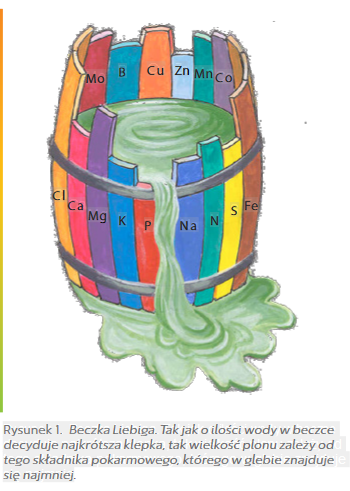
The second important law is the principle of returning nutrients removed from the soil along with plant crops.
To sum up, the ecological and economic justification of fertilization is the adherence to the principle of "as much as necessary, as little as possible" - this also applies to strengthening the soil with nutrients through mineral fertilizers. In order to fertilize in this way, it is necessary to know the abundance of available nutrients in the soil, so perform soil analysis every 4-5 years. Only then are we able to consciously and accurately select the doses of minerals in fertilizers.
Mineral fertilizers - colloquially known as artificial fertilizers - are substances used in plant cultivation to enrich the soil with nutrients.
The first experiments with artificial fertilizers were carried out around 1660 by the German chemist and doctor Johann R. Glauber. He used sodium sulfate, i.e. Glauber's salt.
Mineral fertilizers have been introduced into common use in agriculture mainly thanks to the German chemist Justus Liebig. As a phosphorus fertilizer, Liebig proposed calcium phosphate, obtained by treating ground bones with sulfuric acid.
As early as 1842, the Englishman John B. Lawes patented an industrial method of obtaining phosphorus fertilizer, called superphosphate. Another artificial fertilizer was potassium nitrate, obtained from the then discovered rich deposits, the so-called Chilean saltpetre. With a view to becoming independent from these supplies, at the beginning of the 20th century, the search for technical methods of obtaining nitric acid using atmospheric nitrogen began. In 1905, the first nitrate plant was built in Italy.
The first synthetic nitrogen fertilizer was ammonium sulphate, obtained from 1890 in Germany as a by-product in coking plants. Also in Germany in 1905 the production of calcium cyanamide (CaCN2), known as nitride, began. In 1902 in Norway, Birkeland and Eyde, and in 1903 in Poland, Mościcki developed a method for the synthesis of nitrogen oxide and nitric acid by oxidizing nitrogen from the air in an electric arc. This method, which requires a very large amount of energy, is only used by YARA.
The basis of the current production of nitrogen fertilizers is the synthesis of ammonia developed in Germany in 1913 by Haber and Bosch. The authors of this achievement were honored with Nobel prizes: Haber in 1918 and Bosch in 1935. In 1921, a method for the synthesis of urea from ammonia was also developed in Germany.
As can be seen from this short review, the history of modern agricultural chemistry is almost 170 years old, and its greatest achievements affecting mineral fertilizers date back to the turn of the 19th and 20th centuries.
Mineral fertilizers are divided into:
Basic agrotechnical treatments increasing the effectiveness of mineral fertilization are:
Irregular phosphorus fertilization accelerates the processes of its regression in the soil, which means that it worsens its digestibility and limits the possibilities of effective supplementation of deficiencies.
Division of phosphorus fertilizers:
The pH of the soil affects the absorption of phosphorus, especially when it is inappropriate for a given soil category. In very acidic soils, at the pH (in 1M KCl) below 4.5 - 5.0, the absorption of phosphorus decreases, which turns into practically insoluble compounds with aluminum, which is toxic to plants. Then the phosphorus shows no effect. The soil with a pH of 5.0 - 6.8 (in 1M KCl) contains the most monovalent H2PO4- ions, the most rapidly absorbed by plant roots. In neutral or alkaline or over-limed soils, the uptake of the bivalent HPO4 ion is much less (about 10 times) at a pH above 6.8. In practice, the effects of phosphorus over-fertilization are not observed, because plants do not tend to take up excessive amounts of phosphorus, as they do in the case of nitrogen and potassium.
For over 20 years, potassium has been the most deficient basic nutrient in Polish agriculture.
However, excess potassium is inadvisable, and even harmful - both for the soil and the plant. Too high doses of potassium cause its excessive accumulation in plants - mainly in green parts and roots (everywhere except seeds) - and being in excess, it worsens the biological, technological and storage value of the crop. Therefore, potassium mineral fertilizers (and not only) must be applied taking into account the soil's nutrient requirements.
Calcium is an essential macronutrient for plants. Calcium compounds are used primarily for liming, i.e. regulating the pH of the soil, and not fertilizing plants. In the soil, within the reach of plant roots, there is from dozen to several dozen tons of CaO/ha.
Magnesium is a very "mobile" element, so it is easily leached into the deeper layers of the soil profile. That is why it should be used regularly. As a rule, the lighter and more acidic the soil, the faster the magnesium is washed out.
The cultivation of sulfur-loving plants, i.e. rapeseed, mustard and other cruciferous plants (including cruciferous vegetables) requires greater attention to the problem of sulfur deficiency and its proper fertilization. In turn, the plowed in rapeseed straw, rich in sulfur, will protect the succeeding plant with this ingredient.
If you have questions about mineral fertilizers that are not answered in this article, please contact us! We are happy to answer your questions and advise which mineral fertilizers will work for your crops.